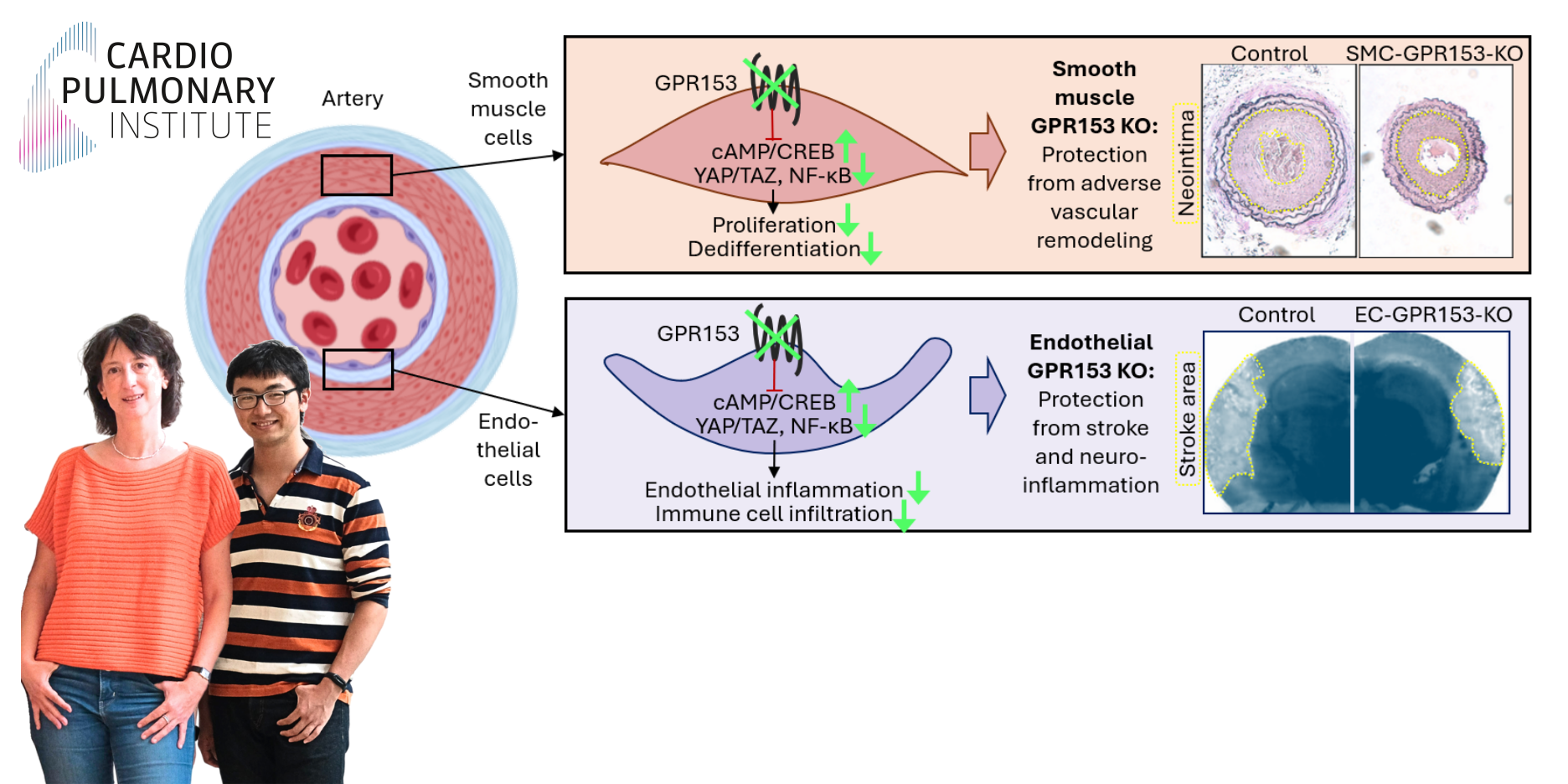
A team of researchers at the Max Planck Institute for Heart and Lung Research has identified a previously uncharacterized role for the orphan G-protein-coupled receptor GPR153 in regulating vascular cell function, inflammation, and remodelling — opening new avenues for therapeutic strategies in vascular disease, neuroinflammation, and stroke.
GPR153, a receptor whose natural ligand and function were unknown, was found to be upregulated in smooth muscle cells (SMCs) following vascular injury. By silencing GPR153, researchers observed reduced SMC proliferation and altered differentiation. “Our study shows that GPR153 is a key switch in smooth muscle biology,” said Jingchen Shao, first author of the study. In mice with SMC-specific inactivation of GPR153, SMC growth after injury was significantly reduced, and pathological neointima development accordingly diminished.
Mechanistically, the study showed that GPR153 serves as a negative regulator of intracellular cAMP. In its absence, cAMP levels rise, leading to increased CREB phosphorylation, suppression of YAP/TAZ signalling, and reduced activation of the inflammatory NF-κB pathway. “On one hand, protective signalling pathways become more active. On the other, a high cAMP level inhibits signalling pathways that promote inflammation and cell division,” explained Nina Wettschureck, group leader at the Max Planck Institute and lead author of the study. These findings suggest that GPR153 acts as a molecular switch promoting both proliferative and inflammatory responses in vascular cells.
Interestingly, similar molecular changes were observed in endothelial cells (ECs), where loss of GPR153 led to decreased expression of inflammatory genes. Together with the group of Markus Schwaninger in Lübeck, the MPI researchers found that mice with EC-specific GPR153 deficiency showed significant improvements in models of multiple sclerosis and ischemic stroke compared to control animals. “We found fewer inflammatory foci in the brains of these mice, less immune cells had migrated in, and the animals showed fewer neurological symptoms,” summarized Shao.
From the perspective of the Max Planck researchers, the study’s findings point to new therapeutic opportunities. “Our data show that GPR153 acts as an amplifier of damaging processes in injured blood vessels, both in smooth muscle and endothelium. Therefore, the next step is to investigate ways to specifically inhibit its upregulation or block its activity following vascular injury,” said Wettschureck.
Find the full article here: https://www.nature.com/articles/s41467-025-61057-w
You need to load content from hCaptcha to submit the form. Please note that doing so will share data with third-party providers.
More InformationYou need to load content from reCAPTCHA to submit the form. Please note that doing so will share data with third-party providers.
More InformationYou need to load content from reCAPTCHA to submit the form. Please note that doing so will share data with third-party providers.
More InformationYou need to load content from reCAPTCHA to submit the form. Please note that doing so will share data with third-party providers.
More InformationYou need to load content from Turnstile to submit the form. Please note that doing so will share data with third-party providers.
More Information